DNA Methylation Analysis of the Hippo signalling Pathway Core Component Genes in Breast Cancer Cells
Main Article Content
Abstract
Objective: Breast cancer is one of the most frequent malignancies among women. According to the World Health Organization (2020), an estimated 2.3 million women were diagnosed with breast cancer, while 685,000 died worldwide. Therefore, an early diagnosis of cancer is crucial for survival. This study analyses the methylation status of the promoter regions of core component genes of the hippo pathway. The Hippo pathway is a tumor suppressor pathway as this pathway hinders cell growth and cell proliferation and motivates cell death.
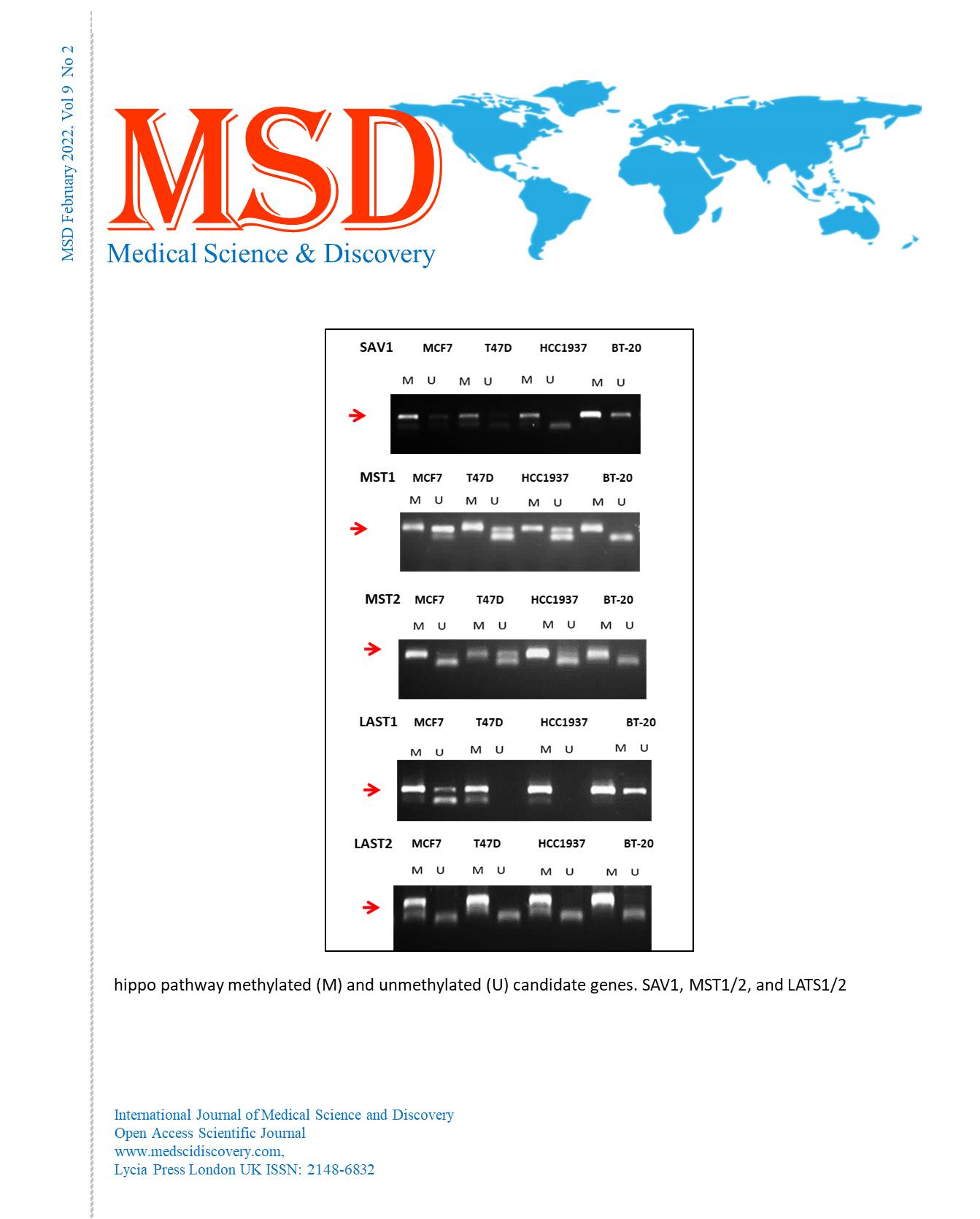
Material and Methods: Methylation-sensitive PCR method was used to examine the altered methylation patterns of SAV1, LAST1/2, and MST1/2 in different breast cancer cell lines (MCF7, T47D, HCC1937, and BT-20).
Results: Interestingly, we have found that the promoter regions of the genes being studied are all hemimethylated in all cell lines used in this investigation, apart from the LAST1 gene promoter, which was hypomethylated in T47D and HCC1937 cell lines.
Conclusion: This indicates the importance of hemimethylation, as it is considered an aberrant methylation pattern. Thus, its effect on gene expression must be further considered.
Downloads
Article Details

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.
Accepted 2022-02-20
Published 2022-02-22
References
Moroishi T, Hansen CG, Guan KL. The emerging roles of YAP and TAZ in cancer. Nat. Rev. Cancer.2015; 15(2): 73–79.
Donninger H, Allen,N, Henson A, Pogue J, Williams A, Gordon L, Kassler S, Dunwell T, Latif F, Clark GJ. Salvador protein is a tumor suppressor effector of RASSF1A with hippo pathway-independent functions. J. Biol. Chem. 2011; 286(21): 18483–18491.
Kai T, Tsukamoto Y, Hijiya N, Tokunaga A, Nakada C, Uchida T, Daa T, Iha H, Takahashi M, Nomura T, Sato F, Mimata H, Ikawa M, Seto M, Matsuura K, Moriyama M. Kidney-specific knockout of Sav1 in the mouse promotes hyperproliferation of renal tubular epithelium through suppression of the Hippo pathway. J. Pathol. 2016; 239(1): 97–108.
Wang L, Wang M, Hu C, Li P, Qiao Y, Xia Y, Liu L, Jiang X. Protein salvador homolog 1 acts as a tumor suppressor and is modulated by hypermethylation in pancreatic ductal adenocarcinoma. Oncotarge.2017; 8(38): 62953–62961.
Yim,SY, Shim JJ, Shin JH, Jeong YS, Kang SH, Kim SB, Eun YG, Lee DJ, Conner EA, Factor VM, Moore DD, Johnson RL, Thorgeirsson SS, Lee JS. Integrated genomic comparison of mouse models reveals their clinical resemblance to human liver cancer. Mol. Cancer Res. 2018;16(11): 1713–1723.
Jiang J, Chang W, Fu Y, Gao Y, Zhao C, Zhang X, Zhang S. SAV1, regulated by microRNA-21, suppresses tumor growth in colorectal cancer. Biochem Cell Biol.2019; 97(2): 91–99.
Ni L, Luo X. Structural and biochemical analyses of the core components of the hippo pathway. Methods Mol. Biol. 2019; 1893: 239–256.
Kim HB, Myung S J. Clinical implications of the Hippo-YAP pathway in multiple cancer contexts. BMB Rep. 2018; 51(3): 119–125.
Dent P, Booth L, Roberts JL, Liu J, Poklepovic A, Lalani AS, Tuveson D, Martinez J, Hancock JF. Neratinib inhibits Hippo/YAP signaling, reduces mutant K-RAS expression, and kills pancreatic and blood cancer cells. Oncogene. 2019; 38(30), 5890–5904.
Sun X, Ding Y, Zhan M, Li Y, Gao D, Wang G, Gao Y, Li Y, Wu S, Lu L, Liu Q, Zhou Z. Usp7 regulates Hippo pathway through deubiquitinating the transcriptional coactivator Yorkie. Nat. Commun.2019; 10(1): 411.
He W, You Y, Du S, Lei T, Wang H, Li X, He H, Tong R, Wang Y. Anti-neoplastic effect of mangiferin on human ovarian adenocarcinoma OVCAR8 cells via the regulation of YAP. Oncol. Lett. 2019;17(1): 1008–1018.
Sudol M., Shields DC, Farooq A. Structures of YAP protein domains reveal promising targets for development of new cancer drugs. Semin Cell Dev. Biol.2012; 23(7): 827–833.
Wei C, Wang Y, Li X. The role of Hippo signal pathway in breast cancer metastasis. Onco Targets and Therapy. 2018;11: 2185–2193.
Bos PD, Zhang XH, Nadal C, Shu W, Gomis RR, Nguyen DX, Minn AJ, van de Vijver MJ, Gerald WL, Foekens JA, Massagué J. Genes that mediate breast cancer metastasis to the brain. Nature. 2009; 459 (7249):1005–1009.
Ehrlich M, Lacey M. DNA hypomethylation and hemimethylation in cancer. Adv. Exp. Med. Biol. 2013;754: 31–56.
Gama-Sosa MA, Slagel, VA, Trewyn RW, Oxenhandler R, Kuo KC, Gehrke CW, Ehrlich M. The 5-methylcytosine content of DNA from human tumors. Nucleic Acids Res. 1983;11(19): 6883–6894.
Romanov GA, Vanyushin BF. Methylation of reiterated sequences in mammalian DNAs. Effects of the tissue type, age, malignancy and hormonal induction. Biochim. Biophys. Acta. 1981; 653(2): 204–218.
Feinberg AP, Vogelstein B. Hypomethylation of ras oncogenes in primary human cancers. Biochem. Biophys. Res. Commun. 1983; 111(1): 47–54.
Feinberg APA, Vogelstein B. Hypomethylation distinguishes genes of some human cancers from their normal counterparts. Nature. 1983; 301(5895): 89–92.
Zhao Z Xiang S, Qi J, We Yi, Zhang M, Yao J, Zhang T, Meng M, Wang X , Zhou Q. Correction of the tumor suppressor Salvador homolog-1 deficiency in tumors by lycorine as a new strategy in lung cancer therapy. Cell Death & Disease. 2020; 11(5):387.
Jin X, Zhu L, Xiao S, Cui Z, Tang J, Yu J, Xie M. MST1 inhibits the progression of breast cancer by regulating the Hippo signaling pathway and may serve as a prognostic biomarker. Molecular Medicine Reports. 2021; 23(5): 383.
Britschgi A, Duss S, Kim S, Couto JP, Brinkhaus H, Koren S, Silva DD, Mertz KD, Kaup D, Varga Z, Vissieres HVA, Leroy C, Roloff T, Stadler MB, Scheel CH, Miraglia LJ, Orth AP, Bonamy GMC, Reddy VA, Bentires-Alj M. The Hippo kinases LATS1 and 2 control human breast cell fate via crosstalk with ERα. Nature. 2017; 541(7638):541-545.
Shao C, Lacey M, Dubeau L, Ehrlich M. Hemimethylation footprints of DNA demethylation in cancer. Epigenetics. 2009; 4(3): 165-175.
Sun S, Lee YR, Enfield B. Hemimethylation Patterns in Breast Cancer Cell Lines. Cancer Informatics. 2019;18: 1176935119872959.
Sun S, Zane A, Fulton C, Philipoom J. Statistical and bioinformatic analysis of hemimethylation patterns in non-small cell lung cancer. BMC Cancer. 2021; 21(1): 268.
Naue J, Lee HY. Considerations for the need of recommendations for the research and publication of DNA methylation results. Forensic Sci Int Genet. 2018; 37: e12-e14.