Histological investigation of the effects of western diet on pressure wound healing period
Main Article Content
Abstract
Objective: The stages of skin wound healing are a dynamic process and it is thought to be related to nutrition. Carbohydrates, proteins and fats have particular importance in different periods of recovery process. Our study has aimed to examine the effects of a western diet with high protein, fat, and carbohydrate content on pressure ulcer healing.
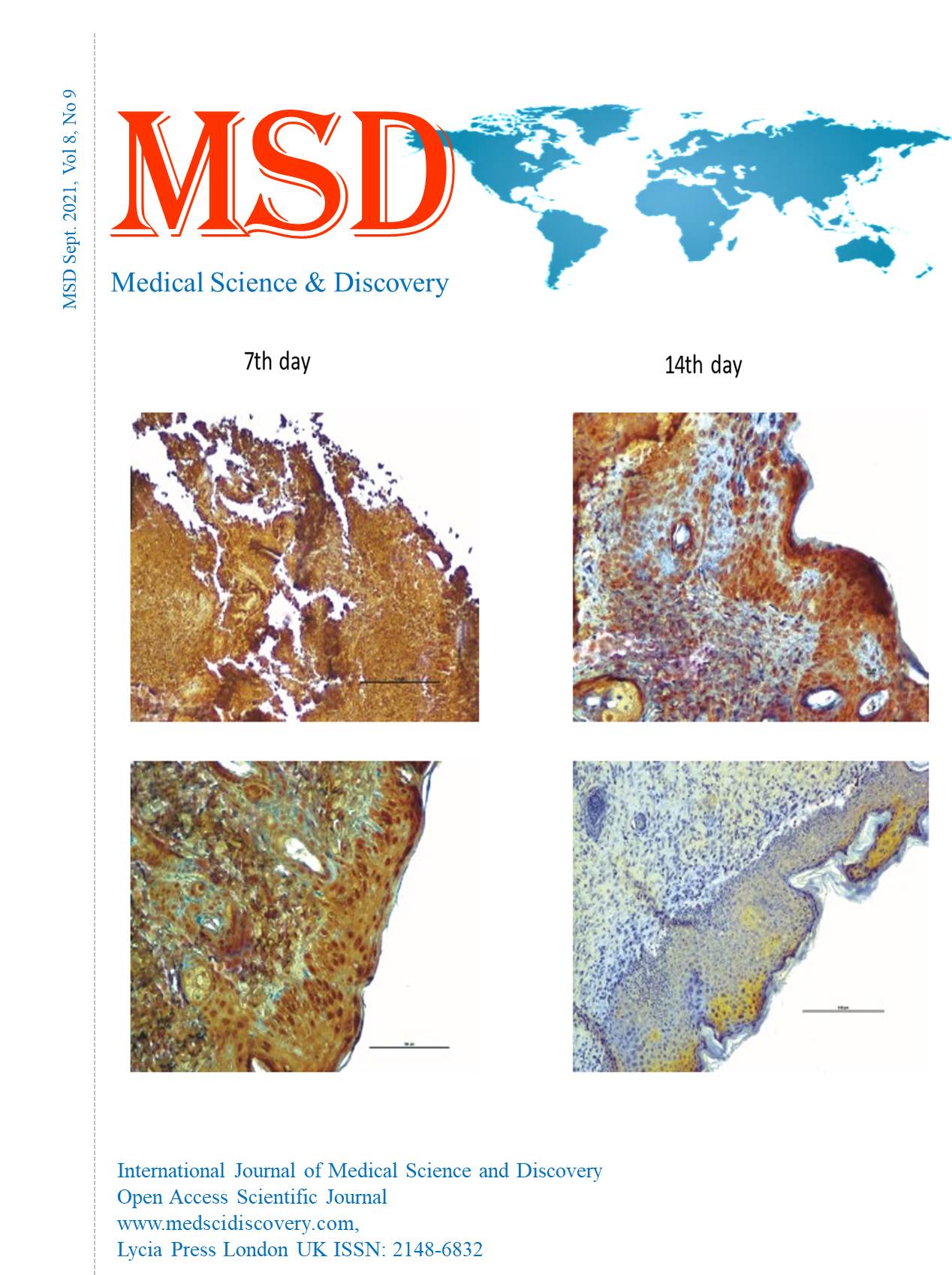
Material and Methods: In this study, we used 22 healthy male Sprague Dawley rats weighing 100-185 g. We randomly divided the rats into two groups. The rats were fed according to the indicated diets (standard diet and western diet). On the first day of the fourth week, ischemia skin by histopathological examinations of the wound tissue samples on the 7th and 14th days of the wound healing period.
Results: Statistically significant differences were observed in histological and immunohistochemical parameters in the tissue samples on the 7th and 14th days. On the 7th day, there were re-epithelialization (P=0.003), granulation cell density (P=0.004), inflammation (P=0.004), and angiogenesis (P= 0.003). We found re-epithelialization (P=0.001), granulation cell density (P=0.002), inflammation (P=0.002), and angiogenesis (P=0.001) on the 14th day. On the 7th and 14th days, we found the p-value between Ki-67 immunohistochemical staining percentages as P= 0.003 and the p-value for VEGF as P=0.002.
Conclusion: We determined that in short-term wound healing, the western type diet was more effective on pressure wound healing than the standard diet.
Downloads
Article Details
Accepted 2021-09-20
Published 2021-09-21
References
Rümeysa Yeniçağ NR. Pressure Ulcers and Nutritional Treatment in Older Adults. Sakarya Medical Journal. 2019;2019;9(3).
Jackson D, Sarki AM, Betteridge R, Brooke J. Medical device-related pressure ulcers: A systematic review and meta-analysis. International journal of nursing studies. 2019;92:109-20.
Dealey C, Posnett J, Walker A. The cost of pressure ulcers in the United Kingdom. Journal of wound care. 2012;21(6):261-2, 4, 6.
Ojeh N, Pastar I, Tomic-Canic M, Stojadinovic O. Stem Cells in Skin Regeneration, Wound Healing, and Their Clinical Applications. International journal of molecular sciences. 2015;16(10):25476-501.
Medlin S. Nutrition for wound healing. British Journal of Nursing. 2012;21(Sup12):S11-S5.
Varlamov O. Western-style diet, sex steroids and metabolism. Biochimica et biophysica acta Molecular basis of disease. 2017;1863(5):1147-55.
Stadler I, Zhang RY, Oskoui P, Whittaker MS, Lanzafame RJ. Development of a simple, noninvasive, clinically relevant model of pressure ulcers in the mouse. Journal of investigative surgery : the official journal of the Academy of Surgical Research. 2004;17(4):221-7.
Anahita Sedighi DM, Reza Shirazi. Histopathological evaluation of the healing effects of human amniotic membrane transplantation in third-degree burn wound injuries. Comparative Clinical Pathology. 2016 >> 25 ( 2) 381-5.
Ulloa-Padilla JP, Ghassibi MP, Dubovy SR, Kerr DA. Clinicopathologic Correlation of Kaposi Sarcoma Involving the Ocular Adnexa: Immunophenotyping of Diagnostic and Therapeutic Targets. Ophthalmic plastic and reconstructive surgery. 2020;36(2):185-90.
Peirce SM, Skalak TC, Rodeheaver GT. Ischemia-reperfusion injury in chronic pressure ulcer formation: a skin model in the rat. Wound repair and regeneration : official publication of the Wound Healing Society [and] the European Tissue Repair Society. 2000;8(1):68-76.
Reid RR, Sull AC, Mogford JE, Roy N, Mustoe TA. A novel murine model of cyclical cutaneous ischemia-reperfusion injury. The Journal of surgical research. 2004;116(1):172-80.
Eming SA, Martin P, Tomic-Canic M. Wound repair and regeneration: mechanisms, signaling, and translation. Science translational medicine. 2014;6(265):265sr6.
Bates DO, Jones ROP. The Role of Vascular Endothelial Growth Factor in Wound Healing. The International Journal of Lower Extremity Wounds. 2003;2(2):107-20.
Brown LF, Yeo KT, Berse B, Yeo TK, Senger DR, Dvorak HF, et al. Expression of vascular permeability factor (vascular endothelial growth factor) by epidermal keratinocytes during wound healing. The Journal of experimental medicine. 1992;176(5):1375-9.
Johnson KE, Wilgus TA. Vascular Endothelial Growth Factor and Angiogenesis in the Regulation of Cutaneous Wound Repair. Advances in wound care. 2014;3(10):647-61.
Leung DW, Cachianes G, Kuang WJ, Goeddel DV, Ferrara N. Vascular endothelial growth factor is a secreted angiogenic mitogen. Science (New York, NY). 1989;246(4935):1306-9.
Pufe T, Paulsen F, Petersen W, Mentlein R, Tsokos M. The angiogenic peptide vascular endothelial growth factor (VEGF) is expressed in chronic sacral pressure ulcers. The Journal of pathology. 2003;200(1):130-6.
Delahunt B, Bethwaite PB, Thornton A, Ribas JL. Proliferation of renal cell carcinoma assessed by fixation-resistant polyclonal Ki-67 antibody labeling. Correlation with clinical outcome. Cancer. 1995;75(11):2714-9.
Key G, Petersen JL, Becker M, Duchrow M, Schlüter C, Askaa J, et al. New antiserum against Ki-67 antigen suitable for double immunostaining of paraffin wax sections. Journal of clinical pathology. 1993;46(12):1080-4.
Sato H, Abe Y, Noguchi M, Kurokawa K, Sakai H. Inhibitory effect of thyrotropic hormone on apoptosis induced by actinomycin D in a functioning rat thyroid cell line. Endocrine journal. 1999;46(2):309-15.
Sayar I, Gelincik I, Bozkurt A, Bayram I. Significance of Ki-67, Bcl-2 and C-erbB2 markers in benign, premalign and malign prostatic lesions/ benign, premalign ve malign prostat lezyonlarinda Ki-67, Bcl-2 ve C-erbB2 belirteclerinin onemi. The Journal of Kartal Training and Research Hospital. 2012;23:123+.
Hopf HW, Gibson JJ, Angeles AP, Constant JS, Feng JJ, Rollins MD, et al. Hyperoxia and angiogenesis. Wound repair and regeneration : official publication of the Wound Healing Society [and] the European Tissue Repair Society. 2005;13(6):558-64.
Güran Ş, Fen T, Tunca Y. Anjiyogenezis ve antianjiyogenik ilaçların kanser tedavisindeki rolü. T Klin Tıp Bilimleri. 2004;24:380-2.
Ramasastry SS. Acute wounds. Clinics in plastic surgery. 2005;32(2):195-208.
Nascimento A, Monte Alto Costa A. Both obesity-prone and obesity-resistant rats present delayed cutaneous wound healing. The British journal of nutrition. 2011;106:603-11.
Paulino do Nascimento A, Monte-Alto-Costa A. Both obesity-prone and obesity-resistant rats present delayed cutaneous wound healing. The British journal of nutrition. 2011;106(4):603-11.
Seitz O, Schürmann C, Hermes N, Müller E, Pfeilschifter J, Frank S, et al. Wound healing in mice with high-fat diet- or ob gene-induced diabetes-obesity syndromes: a comparative study. Experimental diabetes research. 2010;2010:476969.
ÖZKORKMAZ EG, Yusuf Ö. Yara iyileşmesi ve yara iyileşmesinde kullanılan bazı bitkiler. Türk Bilimsel Derlemeler Dergisi. 2009;2(2):63-7.
Flegg JA, Menon SN, Maini PK, McElwain DLS. On the mathematical modeling of wound healing angiogenesis in skin as a reaction-transport process. Frontiers in Physiology. 2015;6(262).
Radomska-Leśniewska DM, Skopiński P, Niemcewicz M, Zdanowski R, Lewicki S, Kocik J, et al. The Effect of Anti-Inflammatory and Antimicrobial Herbal Remedy PADMA 28 on Immunological Angiogenesis and Granulocytes Activity in Mice. Mediators of Inflammation. 2013;2013:853475.
Su WH, Cheng MH, Lee WL, Tsou TS, Chang WH, Chen CS, et al. Nonsteroidal anti-inflammatory drugs for wounds: pain relief or excessive scar formation? Mediators of inflammation. 2010;2010:413238.
Rosa DF, Sarandy MM, Novaes RD, Freitas MB, do Carmo Gouveia Pelúzio M, Gonçalves RV. High-Fat Diet and Alcohol Intake Promotes Inflammation and Impairs Skin Wound Healing in Wistar Rats. Mediators of Inflammation. 2018;2018:4658583.
Baena M, Sangüesa G, Hutter N, Beltrán JM, Sánchez RM, Roglans N, et al. Liquid fructose in Western-diet-fed mice impairs liver insulin signaling and causes cholesterol and triglyceride loading without changing calorie intake and body weight. The Journal of nutritional biochemistry. 2017;40:105-15.
Mayes T, Gottschlich M, Scanlon J, Warden GD. Four-year review of burns as an etiologic factor in the development of long bone fractures in pediatric patients. The Journal of burn care & rehabilitation. 2003;24(5):279-84.
Gottschlich M. Nutrition in the burned pediatric patient. Handbook of Pediatric Nutrition ASPEN. 1999:193-511.